|
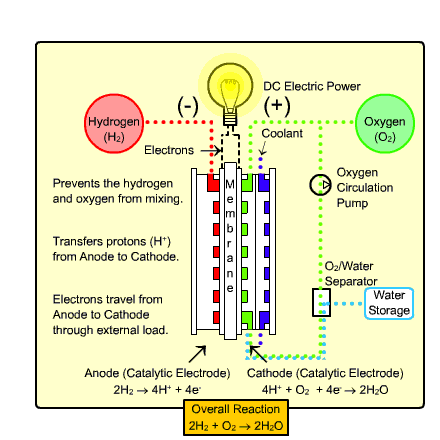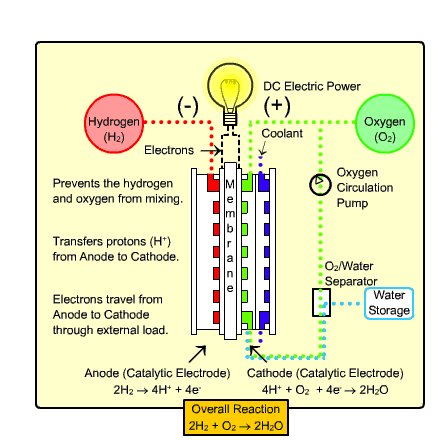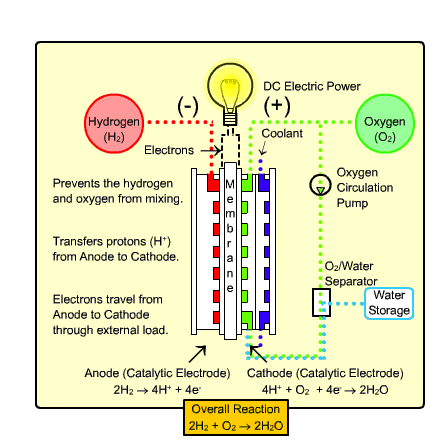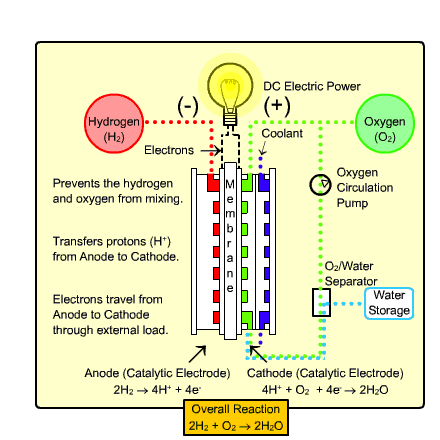
The type of fuel cell that would most likely be used in transportation and the home is called the
Polymer Exchange Membrane Fuel Cell (PEMFC). It warms up quickly due to
its low operating temperature and uses a relatively simple reaction (as shown above).
Process:
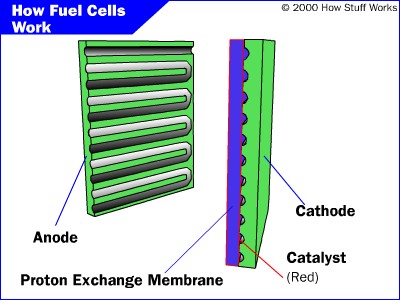
- Hydrogen gas enters the anode side, where it reacts with
the catalyst where it splits into two H+ ions and two electrons.
- Oxygen gas enters the cathode side , where the reaction
with the catalyst forms two oxygen atoms with negative charges.
- The negative charge of the oxygen atoms attracts the hydrogen ions through a Proton
Exchange Membrane while the electrons are forced to go around an external circuit (which creates electricity)
in order to reach the cathode side.
- Two hydrogen atoms, two electrons, and one oxygen atom combine to form a water molecule.
In order to produce enough electricity to power a machine, such as a car, a number of separate fuel
cells must be combined in a fuel-cell stack connected by bipolar plates.
| Parts of a Fuel Cell |
|
|
|
While PEMFC is the fuel cell most likely to be commercialized, there are other existing fuel
cell designs. Two of these are:
- Solid Oxide Fuel Cell (SOFC): With a very high
operating temperature and a long operating life if in continuous use, SOFCs are most suited for large-scale stationary power
generators (ex. in factories).
- Alkaline Fuel Cell (AFC): AFCs have been used in
the U.S. Space Program for 40 years, but require pure hydrogen and oxygen, and are very expensive.
|





